Ayurveda Rasayani
Ayurveda Rasayani is a cGMP Certified Pharmacy. Rasayani a syndicate dedicated to the manufacturing of paramount quality Ayurvedic Medicines for chronic and critical diseases
Quality of medicines plays a vital role in treating any disease
Ayurveda Rasayani is cGMP certified manufacturing facility and has capacity to manufacture and pack various dosage forms like Tablets, Capsules, Powders, Granules, Oils, Ointments etc.
- Medicines keeping Patients Comfort at Core
- Medicines are Palatable
- No Toxicity or Side Effects
- Improves Patient's quality of life
Ayurveda Rasayani
Cancer patients frequently undergo extreme sufferings arising from symptoms of their disease and also a lot of uncertainty due to conventional treatments. Presently available medicines show high degree of toxicity and patients need hospitalization because of parenteral route of administration and also requirement of toxicity management.
This problem has been addressed by Rasayu Cancer Clinic. Our medicines are prepared keeping patients comfort at core. All our medicines are to be administered orally and hence no hospitalisation is needed. Our medicines are packaged in capsules thus not only standardizing the dose but also making it easy to consume. All our medicines are very palatable and hence patients actually enjoy taking the medicines. Moreover, none of our medicines show any toxicity or side effects, rather data demonstrates that it improves patient’s quality of life within a few days of starting our medicines.
Medicines manufactured at this facility are also used by other Ayurveda Physicians for dipensing purpose. Medicines are also exported to USA, Europe & Middle Eastern countries.
Recently we conducted a study to evaluate the perception of our patients regarding ease and convenience of taking medicine. We found our patients feel that Rasayu medicines are very convenient to take and do not have any issues related to palatability. Patients also found Rasayu medicines to be free from problems associated with conventional cancer therapy like intravenous administration, need for hospitalisation, and side effects.
Similarly, patients are advised some Panchakarma procedures. These procedures have been develped in such a way that patients can administer them in the comfort of their homes without help.
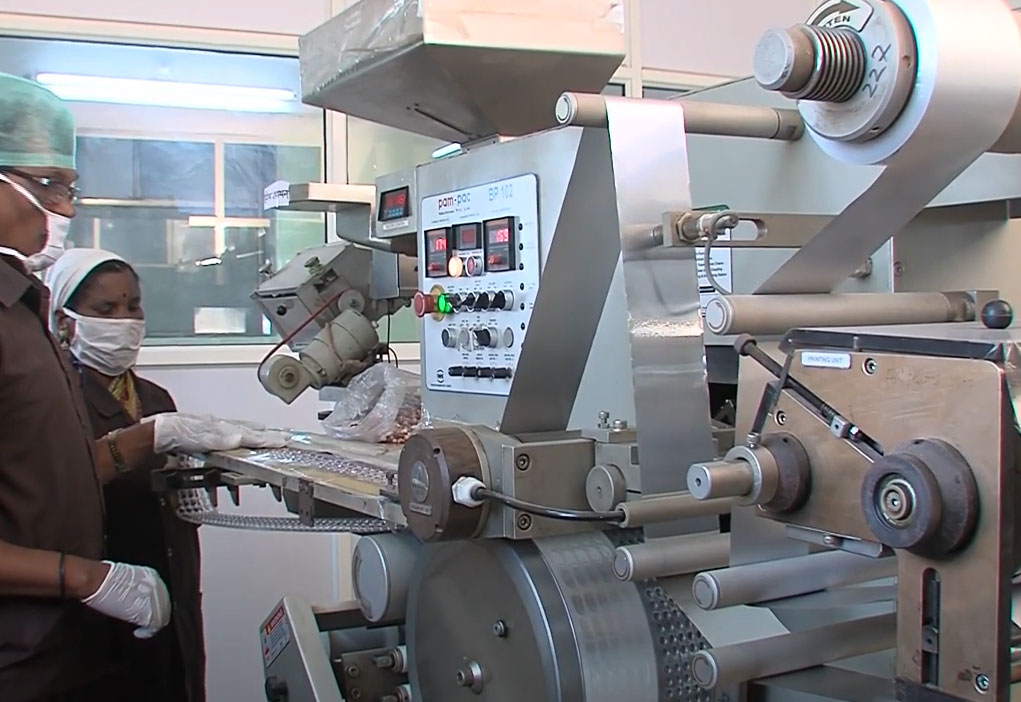

Our Manufacturing Facility
The quality of medicines plays a vital role in treating any disease. If medicine quality is compromised, it may not achieve the desired therapeutic results. It becomes all the more important when it comes to treating critical disease like cancer.
We take utmost care to assure that each and every formulation received by our patient fulfils stringent quality control norms. For assuring this we have developed our own manufacturing facility on the outskirts of Pune. This facility is certified for Good Manufacturing Practice (cGMP). We have advanced machinery for manufacturing medicines and sophisticated ultramodern instruments to analyse them.
Our quality control lab applies the latest tests to analyse these products for quality standards. Each batch of raw material is checked for highest quality standards before it is further processed. All finished products also undergo testing to maintain quality norms. All these efforts lead to production of medicines that meet high standards to treat our patients.
The manufacturing of medicines is done as described in the Ayurveda texts and yet it adheres to the latest good manufacturing practise norms. This leaves no lacunae in the manufacturing process; either in the modern context or in the process prescribed by the texts.












